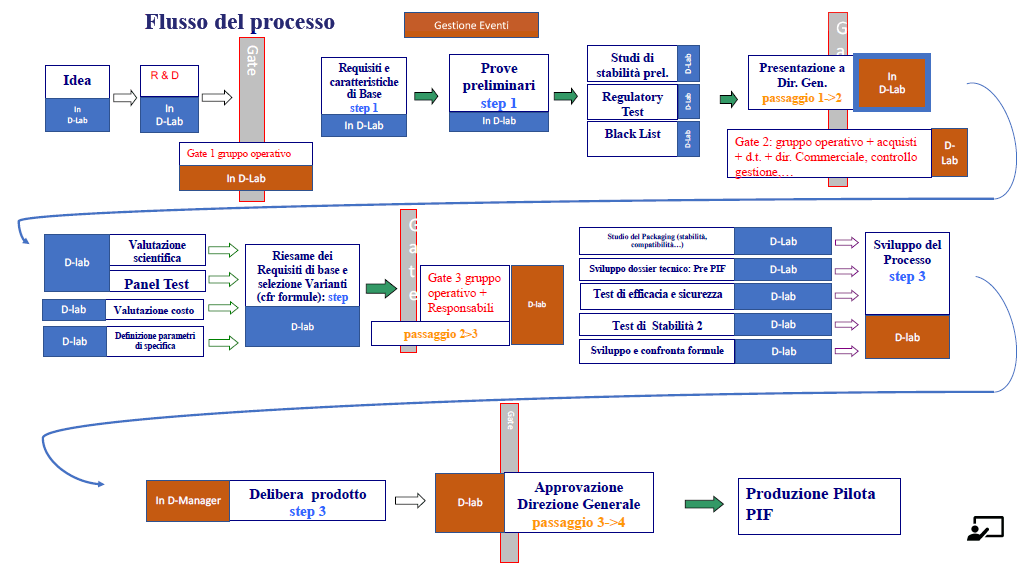
Dedicated to the Research and Development area of the cosmetic industry: the D-Lab module is designed specifically for those who want to start from their new idea and realize it, step by step, in a new winning formula.
In addition to containing all the Regulatory tools of Dossier Manager, D-Lab adds, for the maximization of the R&D process, specific functionalities such as the possibility to create preliminary PIFs, the possibility to create multiple product variants and underlying versions of formulas, the possibility to compare formulas, the possibility to create Blacklists by geographic area or by customer and much more.
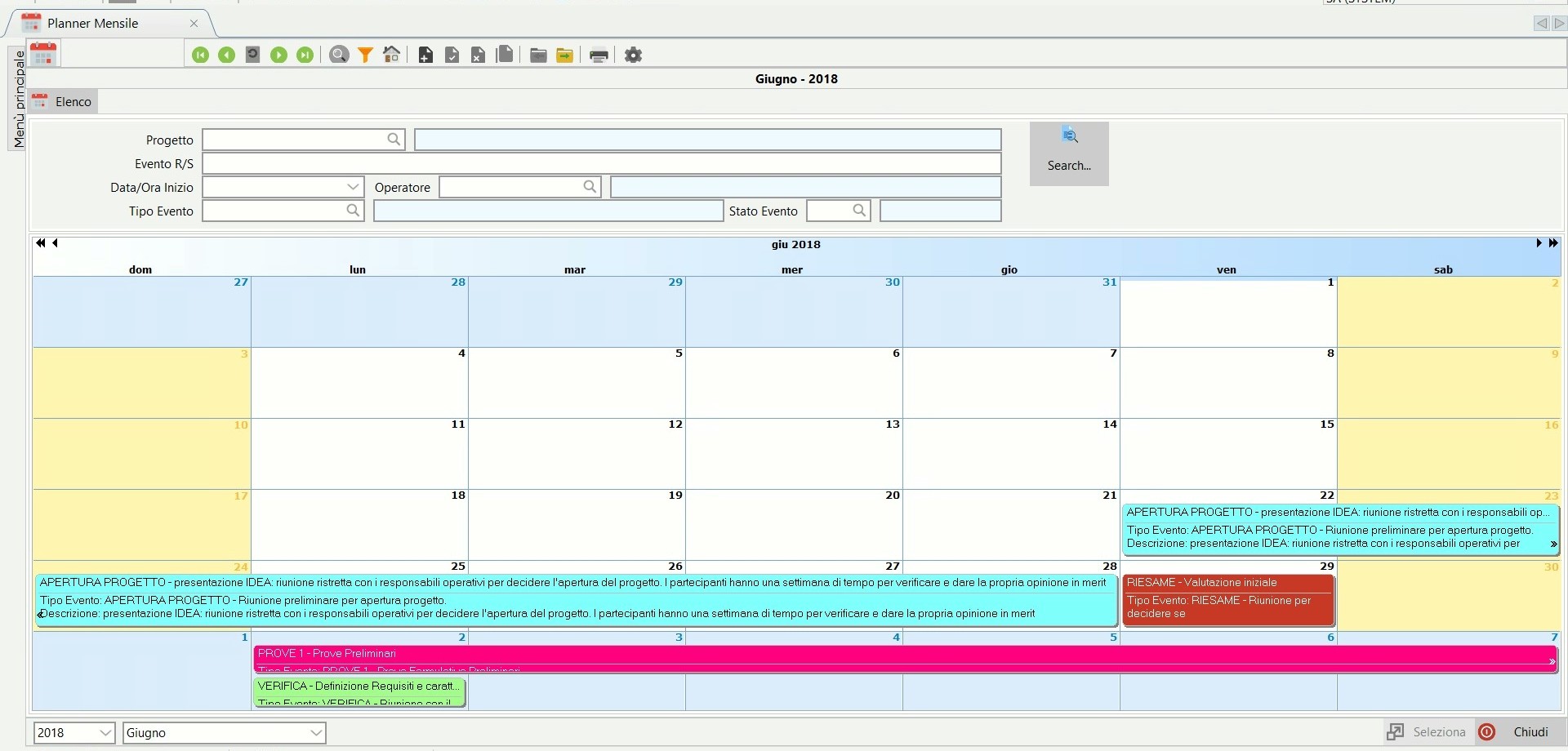
The Planner.
The Project Planner allows you to manage all the activities assigned to the resources involved in a simple and intuitive way.
The planner can be organized by month, operator, project or activity.
These activites are recorded and monitored:
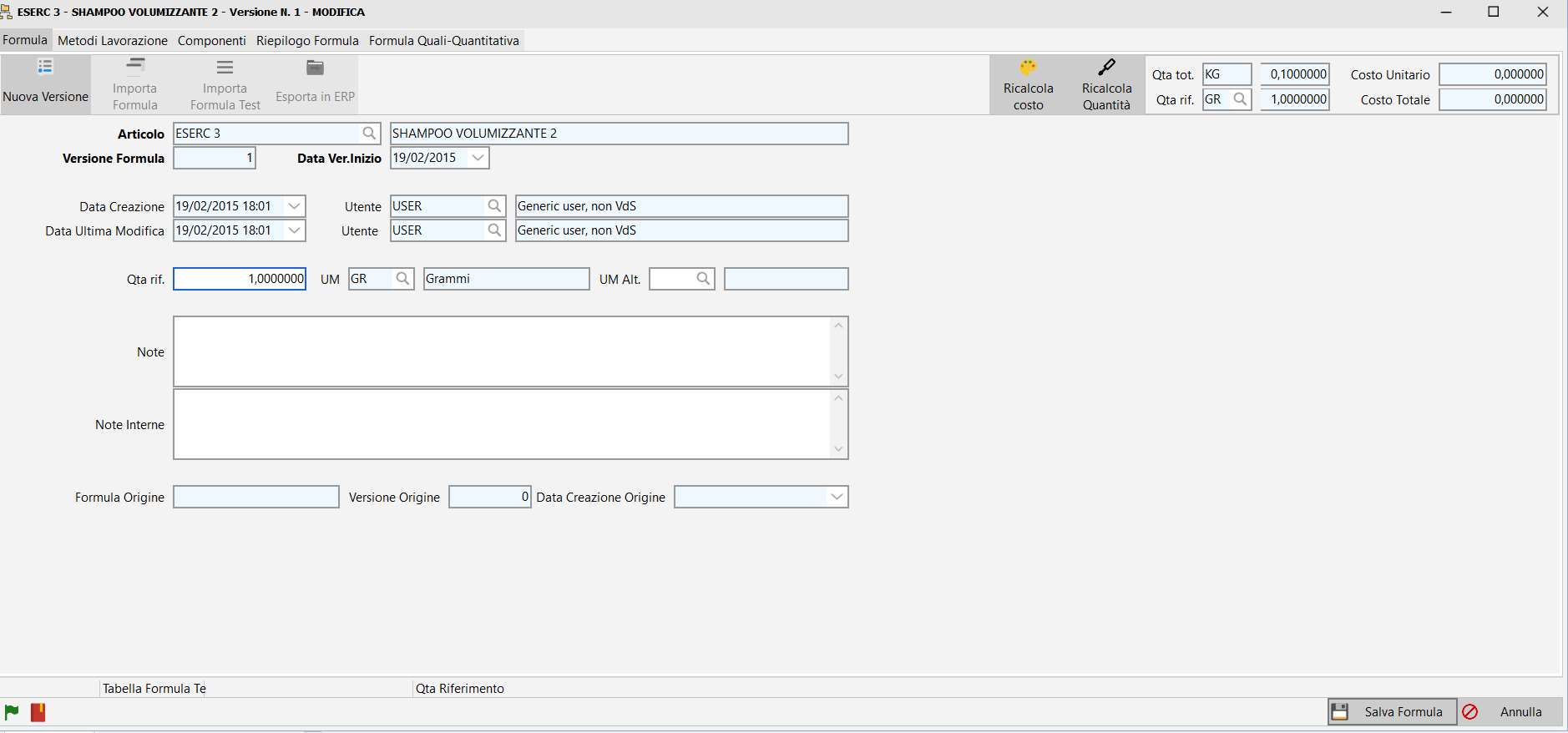
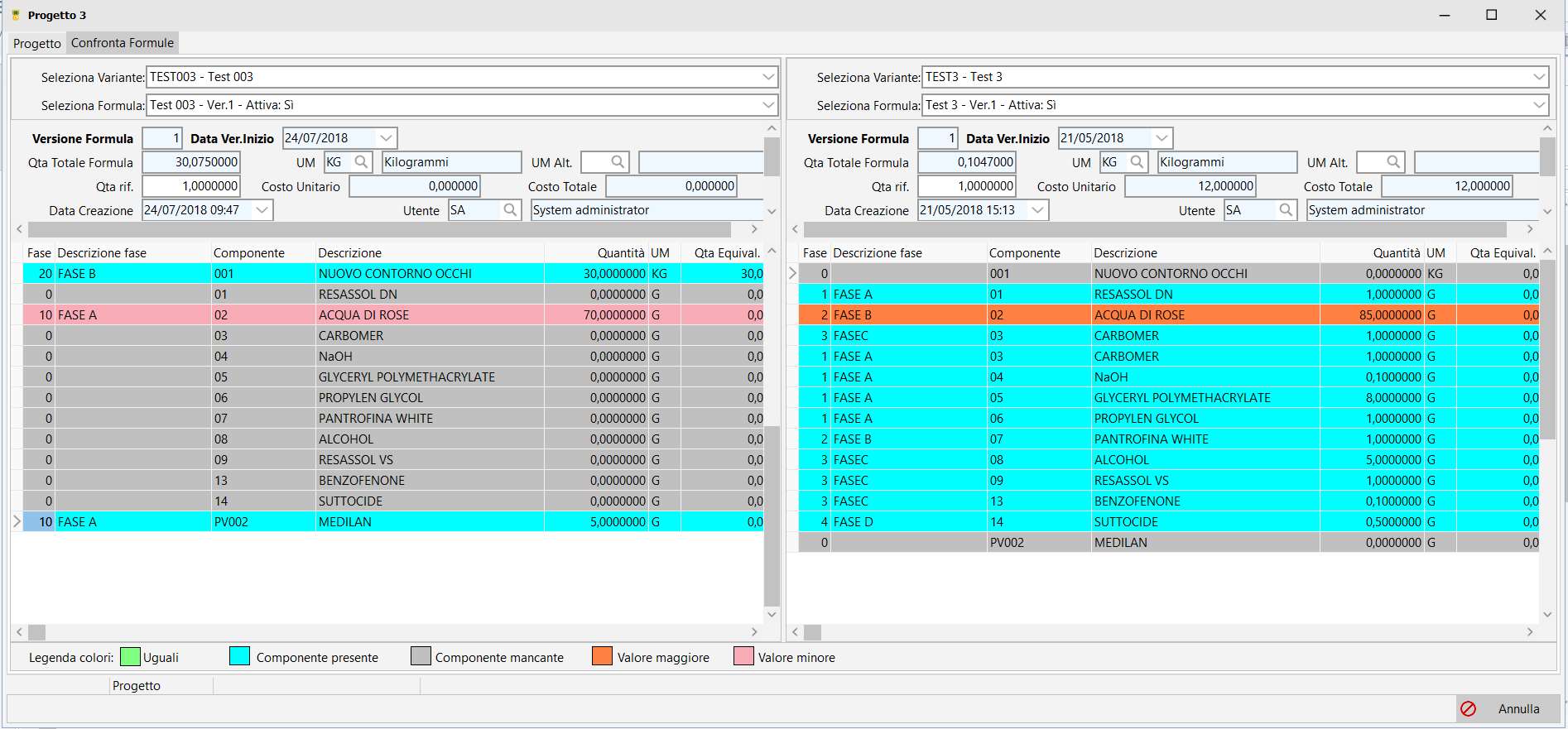
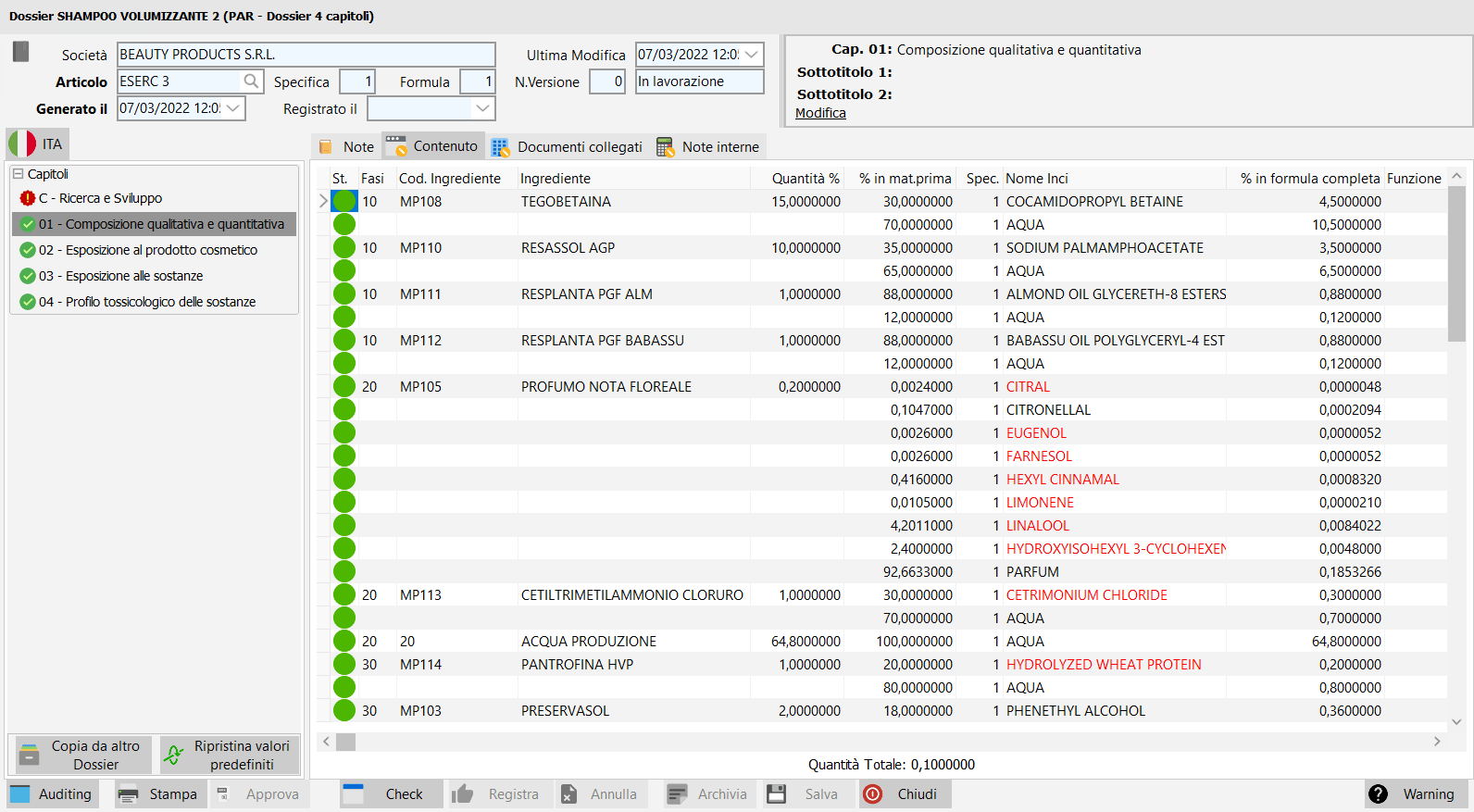
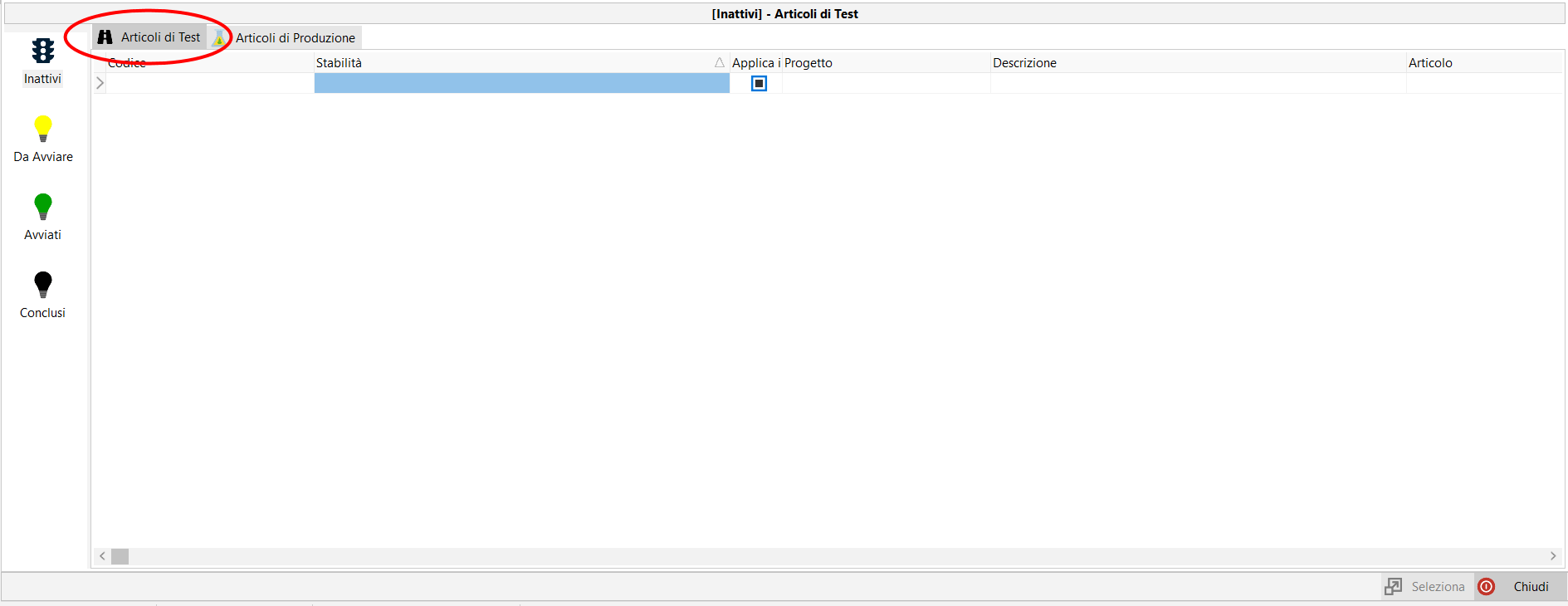
Formula management.
Each project can have multiple variants, each variant containing multiple versions of formulas.
Each new formula can be created from any previous formula, not necessarily the most recent one.
In each variant the currently active formula can be defined.
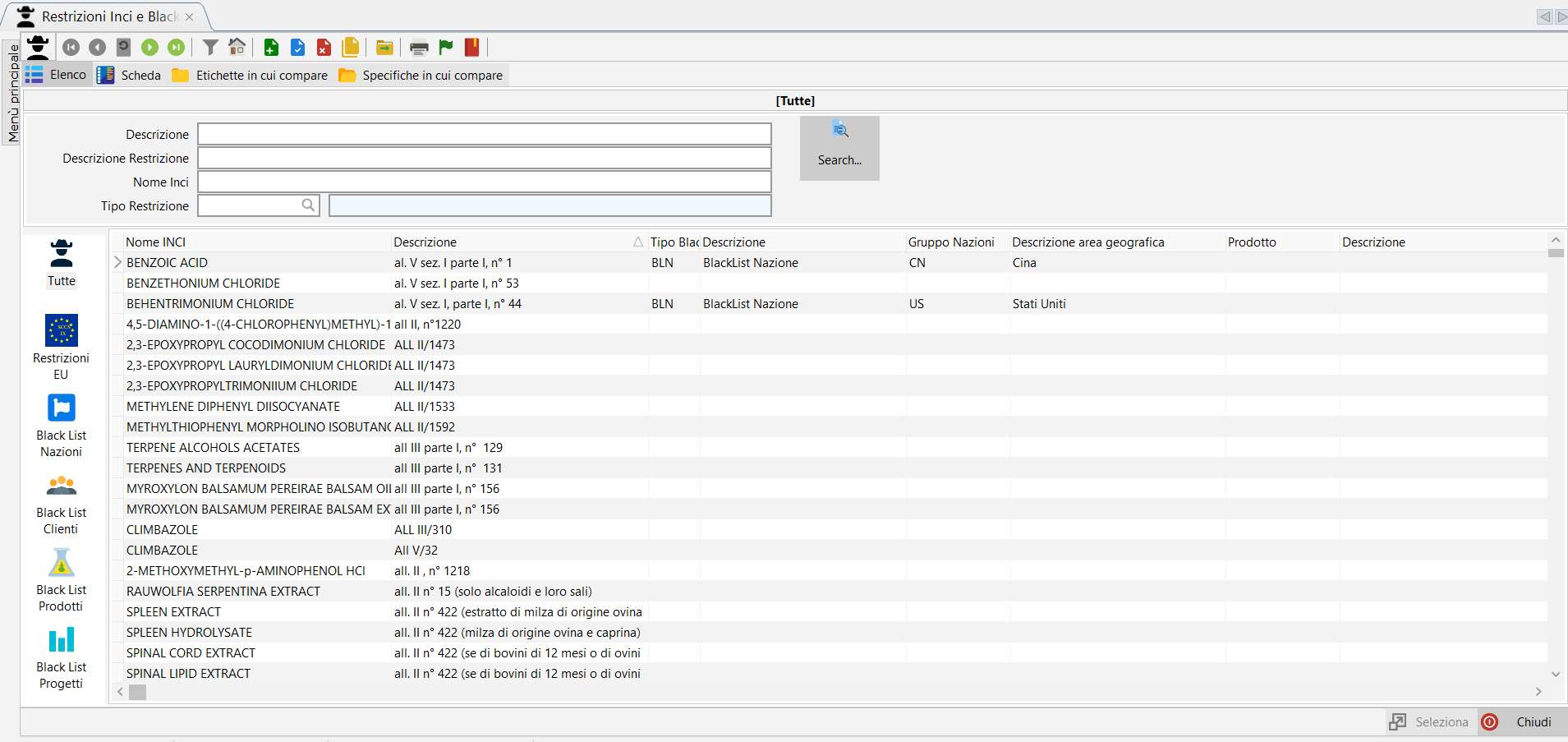
Black List.
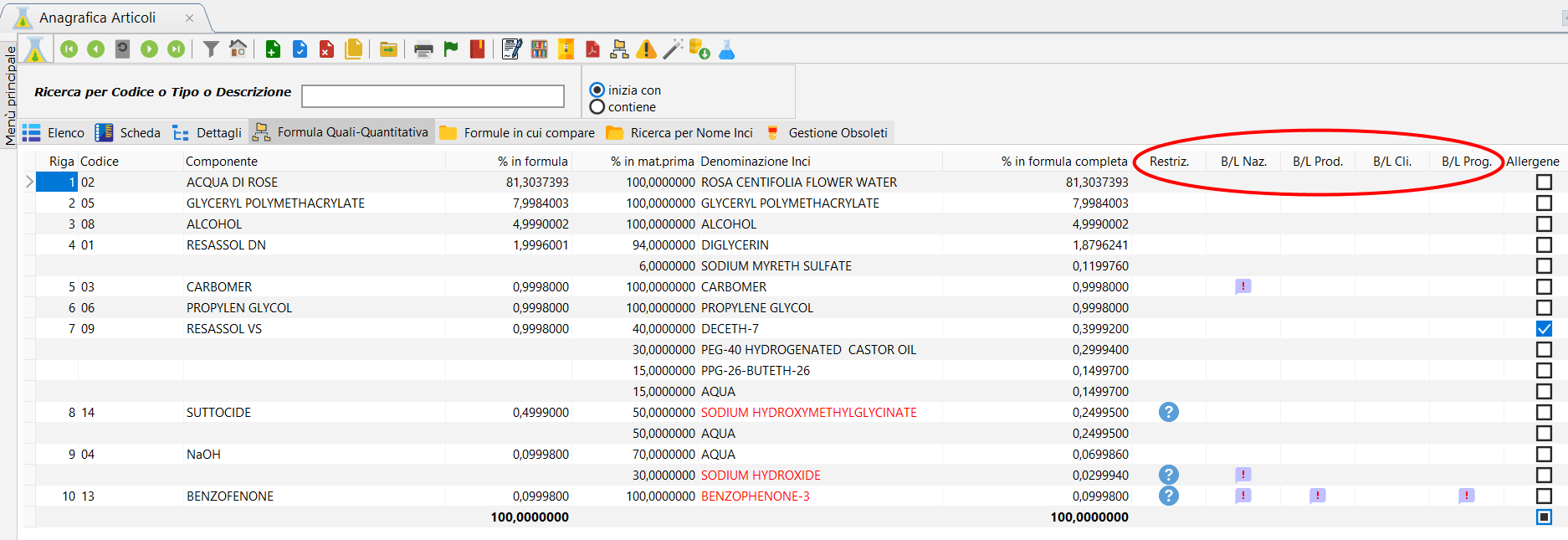
Normally Dossier Manager is in charge of managing INCI Restrictions within the European legislation.
But often there is the need to indicate other types of restrictions, the so-called Black List. These Black List are determined by business decisions and by parties other than the European community such as foreign nations, customers or particular suppliers.
Black List generation from templates.
In order to facilitate the management of black lists, the concept of template has been introduced. Starting from release 8.0.0.0 it is possible to generate and/or modify a black list starting from a template pre-loaded by the user.
Through the Black List Generator, you can create/add Black Lists from templates, modify the % limits of the substances present in the Black List and eventually delete an entire black list or only some of its elements. The possibility of intervening on each single Black List element from the INCI and Black List Restrictions menu remains unchanged.
Regulatory Tests.
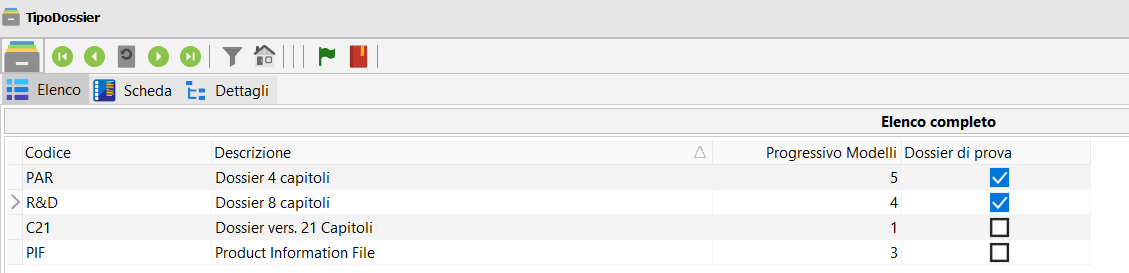
Create a preliminary study or pre-PIF to perform a regulatory test during the R&D process.
You can choose from several pre-prepared Dossier formats, differentiated according to the desired level of study, for example, four or eight chapters.
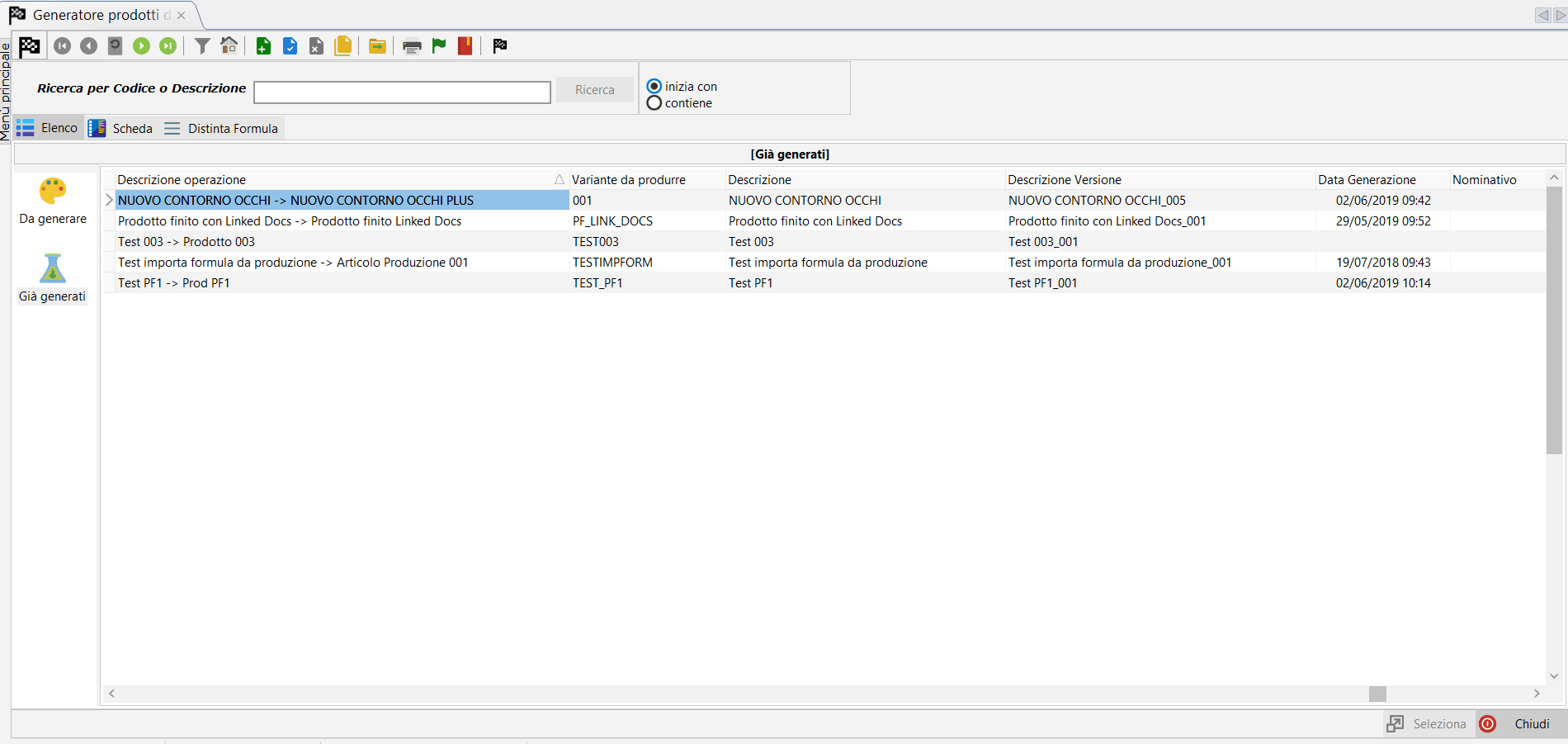
Promotion of the formula.
Once the research and development process is complete, you will then have arrived at the final formula. At this point you can promote that formula to a production item.
At the end of the process one or more winning formulas will move to the production phase with all the dowry of documentation collected and all the technical data.
All information entered by the R&D team will be immediately available to the regulatory team for PIF creation.
D-Lab Exercise at the University of Pavia.
Each year, as part of our Academy Project, at the Master of II level in Cosmetological Sciences of the University of Pavia the Dossier Manager exercise takes place.
The usual appointment brought, with the help of our method, a new class of future professionals to strengthen their advanced theoretical and practical knowledge in the field of legislation, technology, control and evaluation of cosmetic products.